
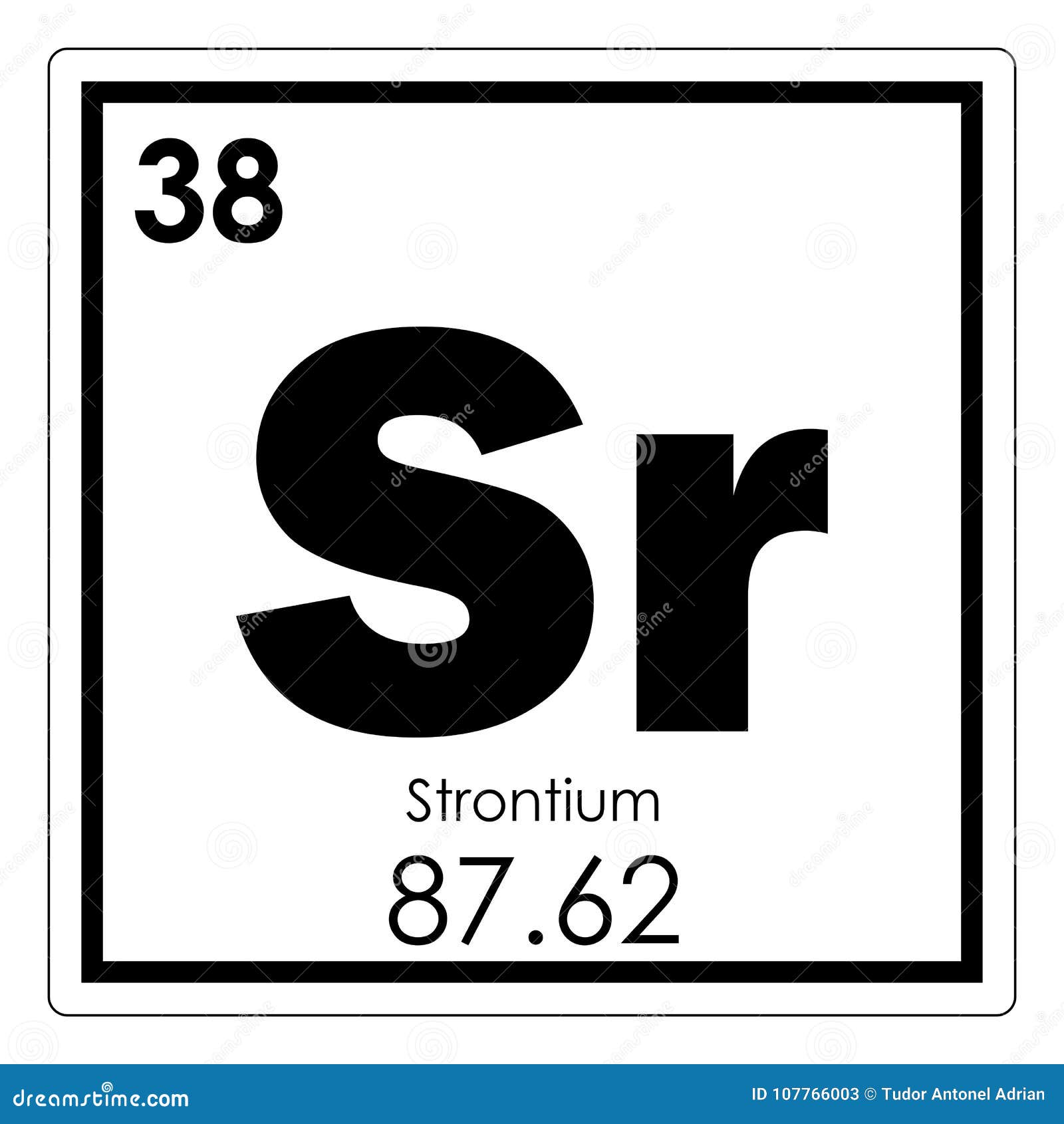
How do you write the electron configuration for Strontium? Seamlessly looping 3D animation on blue illuminated atom design background orbiting electrons name. The electronic configuration of Strontium will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. Description: Strontium as Element 38 of the Periodic Table. What is the electronic configuration of Strontium 38? What is the boiling Point of Strontium in Kelvin?īoiling Point of Strontium in Kelvin is 1655 K. Melting Point of Strontium in Kelvin is 1050 K. What is the melting Point of Strontium in Kelvin? What is the boiling Point of Strontium?īoiling Point of Strontium is 1655 K. Strontium has 38 electrons out of which 2 valence electrons are present in the 5s2 outer orbitals of atom. How many valence electrons does a Strontium atom have? Cruikshank in year 1787 in United Kingdom. The element Strontium was discovered by W. It is located in group 2 and period 5 in the modern periodic table. Strontium is the 38 element on the periodic table. Strontium is a chemical element with the symbol Sr and atomic number 38. What is the position of Strontium in the Periodic Table? aluminum (Al) carbon (C) sulfur (S) tin (Sn), Describe the trends in atomic radii by checking the correct box. Strontium is a chemical element with symbol Sr and atomic number 38. Study with Quizlet and memorize flashcards containing terms like Use the periodic table to determine which elements are likely to have a larger atomic radius than silicon (Si). To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. Sr Strontium Element 38 of Periodic table is Strontium with atomic number 38, atomic weight 87.62. The abbreviated electronic configuration of Strontium is 5s2. What is the abbreviated electronic configuration of Strontium? Alkaline earth metal with various industrial uses, such as in certain. The electronic configuration of Strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2. Strontium, Sr, periodic table element with name, symbol, atomic number and weight.
What is the electronic configuration of Strontium? Strontium Thermal Properties - Enthalpies and thermodynamics Optical Properties of Strontium Refractive IndexĪcoustic Properties of Strontium Speed of Sound Strontium Magnetic Properties Magnetic Type Strontium Heat and Conduction Properties Thermal Conductivity Refer to table below for the Electrical properties ofStrontium Electrical Conductivity Hardness of Strontium - Tests to Measure of Hardness of Element Mohs Hardness Refer to below table for Strontium Physical Properties DensityĢ.63 g/cm3(when liquid at m.p density is $6.98 g/cm3)


 0 kommentar(er)
0 kommentar(er)
